Sunday, December 11, 2011
Friday, December 9, 2011
P6 student objectives sheet
P6 student objectives sheet >
[cid:image001.jpg@01CCB4BE.E3310BD0] 
[cid:image001.jpg@01CCB4BE.E3310BD0]

P6 IGCSE Physics Student Objectives.doc Download this file
Wednesday, December 7, 2011
improvement plan

Improvement Plan - A4.doc Download this file
Monday, November 21, 2011
5.19 Boyle's Law
5.19 Boyle's Law28 October 201111:11·5.19 use the relationship between the pressure and volume of a fixed mass of gas at constant temperature:p1V1 = p2V2
p1 = Pressure at the beginning [kPa, bar or atm]V1 = Volume at the beginning [m3or cm3]p2 = Pressure at the end [kPa, bar or atm]V2 = Volume at the end [m3 or cm3]
(Note: can use any units for V and p as long as they are the same at the beginning and end)
5.19 Boyle's Law
5.19 Boyle's Law demos02 November 201120:01
Fun with the vacuum pump!
· Marshmellows· Food colouring in pipettes· Surgicalgloves
5.19 Experiment07 November 201114:32


· Change the pressure of a fixed mass of gas at a constant temperature· Measure the volume· Use the EXCEL spreadsheet to analyse your results

Boyle's Law.xlsx Download this file
5.18 Answer
5.18 Answer07 November 201115:08Collins, p.116
a. If we cool the gas in a rigid, sealed tin can, what happens to the pressure inside the can? (1 mark)The pressure will decrease
b. Explain your answer to part a. by using the Kinetic Theory (4 marks)· The volume remains the same because a tin can is a rigid container· If the temperature decreases the gas molecules have a lower average Kinetic Energy· So there are less collisions between the gas molecules and the walls of the container per second(NB: Can also say that slower molecules will collide with less force) (1 mark)
· So the pressure in the container will decrease
5.16 Virtual Experiment
5.16 Virtual Experiment28 October 201111:11·5.16 understand that the Kelvin temperature of the gas is proportional to the average kinetic energy of its molecules


1) The variable that remains constant for the experiment is the amount of the gas and the volume of the container.
2) When the temperature is increased the particles have a greater average kinetic energy therefore moving faster so they collide with the walls of the container more frequently with more force increasing the pressure.
3) no, the graph is not a straight line.

4) yes the graph of temperature against (average speed of particles)2 is a straight line.

5) the particles in a container have got a range of speed therefore a range of KEs. Some particles will be moving faster and some slower, but on average, T is proportional to KE.
5.16 Blank EXCEL template07 November 201113:51<<Ideal Gas - temperature vs average KE of particles blank table.xlsx>>

Ideal Gas - temperature vs average KE of particles blank table.xlsx Download this file
Sunday, November 20, 2011
Saturday, November 19, 2011
5.17
5.17 starter
02 November 2011 20:01 > Why do the eggs get sucked into the bottles?! Explanation· The burning paper in the bottle heats the air in the bottle
· When the egg gets placed on top, the oxygen supply in the bottle is rapidly depleted and the paper goes out
· The bottle is sealed by the egg and now has a constant volume of gas inside
· The hot gas in the bottle now starts to cool which reduces the pressure inside the bottle
· The pressure outside the bottle remains unchanged and so there is now an unbalanced force on the egg which accelerates the egg into the bottle 5.17 28 October 2011 11:11
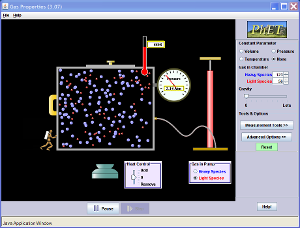
· 5.17 describe the qualitative relationship between pressure and Kelvin temperature for a gas in a sealed container Instructions
· Launch the application on this website: http://phet.colorado.edu/en/simulation/gas-properties [cid:image001.png@01CCA5D2.01AE3110]
· Put 5 pumps of gas in
· Set volume as the Constant Parameter
· Heat to 1000K
· Watch what happens to the Pressure Conclusion
· If you increase the temperature, you increase the pressure 5.17 Demo 02 November 2011 19:56 Cloud formation
· Place a little water in the bottom of a 1½ litre plastic bottle
· Squeeze a few times
· Introduce a small amount of smoke
· Squeeze and release several times
· When you squeeze, the cloud disappears; when you release, the cloud reforms Explanation
· When the pressure increases the temperature increases and vica versa
· The "cloud" is water droplets - liquid water
· When you squeeze the bottle the temperature increases and the droplets turn into water vapour
· The smoke particles are nucleating sites on which the water can condense
Monday, November 14, 2011
5.14
5.1428 October 201111:10·5.14 describe the Kelvin scale of temperature and be able to convert between the Kelvin and Celsius scalesConverting Centigrade to Kelvin
TK = ToC + 273Converting Kelvin to Centigrade
ToC = TK - 273TK = Temperature in Kelvin [K]ToC = Temperature in Degrees Centigrade [oC]5.14 Questions02 November 201118:29· Collins p.118
Q1)
ai) 20+273= 293K
aii) 150+273= 423K
aiii) 1000+273= 1273
bi) 300-273= 27oC
bii) 650-273= 377oC
biii) 1000-273= 723oC
5.13
5.13 Starter02 November 201118:17·How can you fit a giraffe, 2 dogs and a swan into a standard laboratory beaker?!5.13 Starter 202 November 201118:17· Use particle theory to explain why the gas in the balloon contracts
Explanation
· The temperature of the gas inside the balloon decreases so the average speed of the particles decreases· Consequently the gas particles collide with the walls of the balloon with less force and less collisions per second· Because the walls of the container are flexible, the volume decreases5.13 Charles' law28 October 201111:10· 5.13 understand that there is an absolute zero of temperature which is –273oC
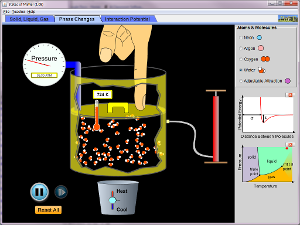
Open the Charles' law interactive experiment
· Adjust the temperature· What’s the relationship between temperature and volume?· Plot a graph of V against T· Take a screen shot of the graph
5.13 results and conclusion28 October 201111:10

Conclusion
· Volume is directly proportional to absolute (Kelvin) temperature· (gas is at constant pressure - flexible container)· V α T5.13 Plenary02 November 201119:13

Instructions
· Keep the Pressure constant (flexible container)· Pump some gas particles in· Predict what will happen when you heat the gas. Try it.· Predict what will happen when you cool the gas. Try it.
Question
· What would happen to the volume of the container if you could cool the gas to absolute zero, 0K? The volume will decrease as the gas cools down.

Charles' law interactive experiment.swf Download this file
5.13
5.13 Starter02 November 201118:17·How can you fit a giraffe, 2 dogs and a swan into a standard laboratory beaker?!5.13 Starter 202 November 201118:17· Use particle theory to explain why the gas in the balloon contracts
Explanation
· The temperature of the gas inside the balloon decreases so the average speed of the particles decreases· Consequently the gas particles collide with the walls of the balloon with less force and less collisions per second· Because the walls of the container are flexible, the volume decreases5.13 Charles' law28 October 201111:10· 5.13 understand that there is an absolute zero of temperature which is –273oC
Open the Charles' law interactive experiment
· Adjust the temperature· What’s the relationship between temperature and volume?· Plot a graph of V against T· Take a screen shot of the graph
5.13 results and conclusion28 October 201111:10

Conclusion
· Volume is directly proportional to absolute (Kelvin) temperature· (gas is at constant pressure - flexible container)· V α T5.13 Plenary02 November 201119:13

Instructions
· Keep the Pressure constant (flexible container)· Pump some gas particles in· Predict what will happen when you heat the gas. Try it.· Predict what will happen when you cool the gas. Try it.
Question
· What would happen to the volume of the container if you could cool the gas to absolute zero, 0K?

Charles' law interactive experiment.swf Download this file
Sunday, November 13, 2011
5.11
5.11 Starter 02 November 2011 16:58
>
· You're looking at smoke particles in air under a microscope
· They appear to be jiggling about
· Why?
· (Don't worry if you can't work this out straight away - Albert Einstein was the bloke who eventually explained what's happening here!) 5.11 28 October 2011 11:10
· 5.11 understand the significance of Brownian motion
> Model 1
· What does the red puck represent?
· What do the metal balls represent?
[cid:image001.png@01CC9989.A14EFF60]
[cid:image002.png@01CC9989.A14EFF60]
> Model 3
· What do the "smoke" particles look like?
· Why are they moving?
· What do the "air" particles look like? 5.11 explained 28 October 2011 11:10 Model 1
· What does the red puck represent?
o The large, visible smoke particle
· What do the metal balls represent?
o The small, not visible air particles Model 2
· What do the small red particles represent?
o The small, not visible air particles
· What does the large blue particle represent?
o The large, visible smoke particle
· What does the view on the left of the screen represent?
o The view through the microscope lense
· Why can‘t you see the red particles in this view?
o They are too small to see Model 3
· What do the "smoke" particles look like?
o They are the 5 large, sand coloured particles
· Why are they moving?
o Small, fast moving air particles are colliding with the smoke particles and making them move
· What do the "air" particles look like?
o They are the numerous, small, white particles 5.11 Questions 02 November 2011 17:21
1. Draw the path of a smoke particle in air (3 marks) The smoke particles would be in a random path in the air because it collides randomly with the invisible air particles.
2. Explain what is meant by Brownian Motion of smoke particles in air and how it provides evidence for air particles (4 marks) Brownian motion is the random drifting of the smoke particles suspended in a gas. It provides evidence for the air particles by showing a white particle in the air moving around.
3. What change would you expect to see in the movement of the smoke particles if the air was cooled down? Why? (2 marks) If the air was cooled down the movement of the smoke particles would be slower because there is less energy. 
>
· You're looking at smoke particles in air under a microscope
· They appear to be jiggling about
· Why?
· (Don't worry if you can't work this out straight away - Albert Einstein was the bloke who eventually explained what's happening here!) 5.11 28 October 2011 11:10
· 5.11 understand the significance of Brownian motion
> Model 1
· What does the red puck represent?
· What do the metal balls represent?
[cid:image001.png@01CC9989.A14EFF60]
[cid:image002.png@01CC9989.A14EFF60]
> Model 3
· What do the "smoke" particles look like?
· Why are they moving?
· What do the "air" particles look like? 5.11 explained 28 October 2011 11:10 Model 1
· What does the red puck represent?
o The large, visible smoke particle
· What do the metal balls represent?
o The small, not visible air particles Model 2
· What do the small red particles represent?
o The small, not visible air particles
· What does the large blue particle represent?
o The large, visible smoke particle
· What does the view on the left of the screen represent?
o The view through the microscope lense
· Why can‘t you see the red particles in this view?
o They are too small to see Model 3
· What do the "smoke" particles look like?
o They are the 5 large, sand coloured particles
· Why are they moving?
o Small, fast moving air particles are colliding with the smoke particles and making them move
· What do the "air" particles look like?
o They are the numerous, small, white particles 5.11 Questions 02 November 2011 17:21
1. Draw the path of a smoke particle in air (3 marks) The smoke particles would be in a random path in the air because it collides randomly with the invisible air particles.
2. Explain what is meant by Brownian Motion of smoke particles in air and how it provides evidence for air particles (4 marks) Brownian motion is the random drifting of the smoke particles suspended in a gas. It provides evidence for the air particles by showing a white particle in the air moving around.
3. What change would you expect to see in the movement of the smoke particles if the air was cooled down? Why? (2 marks) If the air was cooled down the movement of the smoke particles would be slower because there is less energy.

brownian_motion.swf Download this file
Thursday, November 10, 2011
Next Physics e-lesson 5.12+5.15
5.12+5.15 Starter 02 November 2011 16:15 > Questions
· Why does the needle on the meter move when gas particles are introduced into the box?
· What does the meter measure? Answers
· The gas particles collide with all of the walls of the container. The wall on the right moves outwards and moves the needle.
· Pressure. The gas particles colliding with the walls makes a force on the walls. The walls have a surface area so the quantity measured is pressure, p=F/A. 5.12+5.15 Questions 02 November 2011 15:55
· 5.12 recall that molecules in a gas have a random motion and that they exert a force and hence a pressure on the walls of the container
· 5.15 understand that an increase in temperature results in an increase in the speed of gas molecules
[cid:image001.png@01CC9986.E18EA0B0] Try the animation http://www.lon-capa.org/~mmp/kap10/cd283.htm
1. How do the particles create a pressure?
2. If you increase the temperature, how does the movement of the particles change?
3. If you increase the temperature, how does the number of collisions per second change?
4. If you increase the temperature, what does this do to the pressure? 5.12+5.15 Plenary 02 November 2011 15:55 > 

· Why does the needle on the meter move when gas particles are introduced into the box?
· What does the meter measure? Answers
· The gas particles collide with all of the walls of the container. The wall on the right moves outwards and moves the needle.
· Pressure. The gas particles colliding with the walls makes a force on the walls. The walls have a surface area so the quantity measured is pressure, p=F/A. 5.12+5.15 Questions 02 November 2011 15:55
· 5.12 recall that molecules in a gas have a random motion and that they exert a force and hence a pressure on the walls of the container
· 5.15 understand that an increase in temperature results in an increase in the speed of gas molecules
[cid:image001.png@01CC9986.E18EA0B0] Try the animation http://www.lon-capa.org/~mmp/kap10/cd283.htm
1. How do the particles create a pressure?
2. If you increase the temperature, how does the movement of the particles change?
3. If you increase the temperature, how does the number of collisions per second change?
4. If you increase the temperature, what does this do to the pressure? 5.12+5.15 Plenary 02 November 2011 15:55 >

Ideal gases - summary of terms.pptx Download this file

Tuesday, November 1, 2011
5.7 and 5.8
Instructions for Objective 5.7 and 5.8 1. 5.7 and 5.8 Starter. Find out the names of the processes. Research on the internet if necessary. No need to blog this. 2. 5.7 and 5.8. Forward this e-mail to your blog and type the answers into the e-mail. 3. 5.7 and 5.8 Experiment. I’m afraid you can’t do the expt until we get back but watch the video clip to see how it’s set up and have a look at the graph of the results. 4. 5.7 to 5.10 Plenary 1. Play the attached “States of Matter” 5. 5.7 to 5.10 Plenary 2. Play the attached “Fill the trucks” 6. PhET States of matter simulation - embedding into your Posterous blog. Embed in your blog and then have a play 5.7 and 5.8 Starter 28 October 2011 11:00
· What are the 6 processes shown by the arrows?
[cid:image001.png@01CC9575.87F11DE0] 5.7 and 5.8 28 October 2011 10:20
· 5.7 understand that a substance can change state from solid to liquid by the process of melting
· 5.8 understand that a substance can change state from liquid to gas by the process of evaporation or boiling
· Questions from Collins p.112
· Answer in Bullet Points!
[cid:image002.png@01CC9575.87F11DE0]
[cid:image003.png@01CC9575.87F11DE0]
· Use following pages from Collins as a resource to help you
[cid:image019.jpg@01CC9576.07199B60]
[cid:image020.jpg@01CC9576.07199B60]
[cid:image021.jpg@01CC9576.07199B60]
[cid:image022.jpg@01CC9576.07199B60]
[cid:image023.jpg@01CC9576.07199B60] 5.7 and 5.8 Experiment - Cooling Curve of Stearic Acid using datalogger 15 October 2010 14:34
[cid:image024.jpg@01CC9576.07199B60]
5.7 to 5.10 Plenary 1 28 October 2011 12:19 · Play the Stage 1 game to test your knowledge of solids, liquids and gases
· Play the Stage 2 game to test your knowledge about changes of phase! 5.7 to 5.10 Plenary 2 28 October 2011 12:19
Play the Level 1 game to test your knowledge of the properties of solids, liquids and gases Extension: Play the Level 2 game to extend your knowledge about changes of phase! PhET States of matter simulation - embedding into your Posterous blog 28 October 2011 11:14
· Create a post
[cid:image016.png@01CC9575.87F11DE0]
· Turn on HTML editor
[cid:image017.png@01CC9575.87F11DE0]
· Copy in this text and Publish
· Success! Now have a play with the simulation...
[cid:image018.png@01CC9575.87F11DE0] 




· What are the 6 processes shown by the arrows?
[cid:image001.png@01CC9575.87F11DE0] 5.7 and 5.8 28 October 2011 10:20
· 5.7 understand that a substance can change state from solid to liquid by the process of melting
· 5.8 understand that a substance can change state from liquid to gas by the process of evaporation or boiling
· Questions from Collins p.112
· Answer in Bullet Points!
[cid:image002.png@01CC9575.87F11DE0]
[cid:image003.png@01CC9575.87F11DE0]
· Use following pages from Collins as a resource to help you
[cid:image019.jpg@01CC9576.07199B60]
[cid:image020.jpg@01CC9576.07199B60]
[cid:image021.jpg@01CC9576.07199B60]
[cid:image022.jpg@01CC9576.07199B60]
[cid:image023.jpg@01CC9576.07199B60] 5.7 and 5.8 Experiment - Cooling Curve of Stearic Acid using datalogger 15 October 2010 14:34
[cid:image024.jpg@01CC9576.07199B60]
5.7 to 5.10 Plenary 1 28 October 2011 12:19 · Play the Stage 1 game to test your knowledge of solids, liquids and gases
· Play the Stage 2 game to test your knowledge about changes of phase! 5.7 to 5.10 Plenary 2 28 October 2011 12:19
Play the Level 1 game to test your knowledge of the properties of solids, liquids and gases Extension: Play the Level 2 game to extend your knowledge about changes of phase! PhET States of matter simulation - embedding into your Posterous blog 28 October 2011 11:14
· Create a post
[cid:image016.png@01CC9575.87F11DE0]
· Turn on HTML editor
[cid:image017.png@01CC9575.87F11DE0]
· Copy in this text and Publish
· Success! Now have a play with the simulation...
[cid:image018.png@01CC9575.87F11DE0]

states of matter drag and drop plenary.swf Download this file

Fill the trucks - Properties of s,l,g.swf Download this file



Subscribe to:
Comments (Atom)